ISO 13485 Certification In Singapore
ISO 13485 Certification In Singapore
ISO 13485 Certification in Singapore, We are one of the leading ISO Certification providers in Singapore. Factocert provides ISO Consultant service in Hougang, Tampines, Pasir Ris, Yishun, Choa Chu Kang, Toa Payoh, Bukit Batok, Clementi, Jurong, Sengkang, and other major cities.
ISO 13485 Certification in Singapore covers all the aspects of commercial business, and industrial sectors, ISO 13485 certification services in Singapore deals with the food safety and management system. Implementation of ISO 13485 registration services in Singapore involves the high-level structure which made it easier for integration with other international standards.
What are the steps to get ISO 13485 Certification in Singapore:
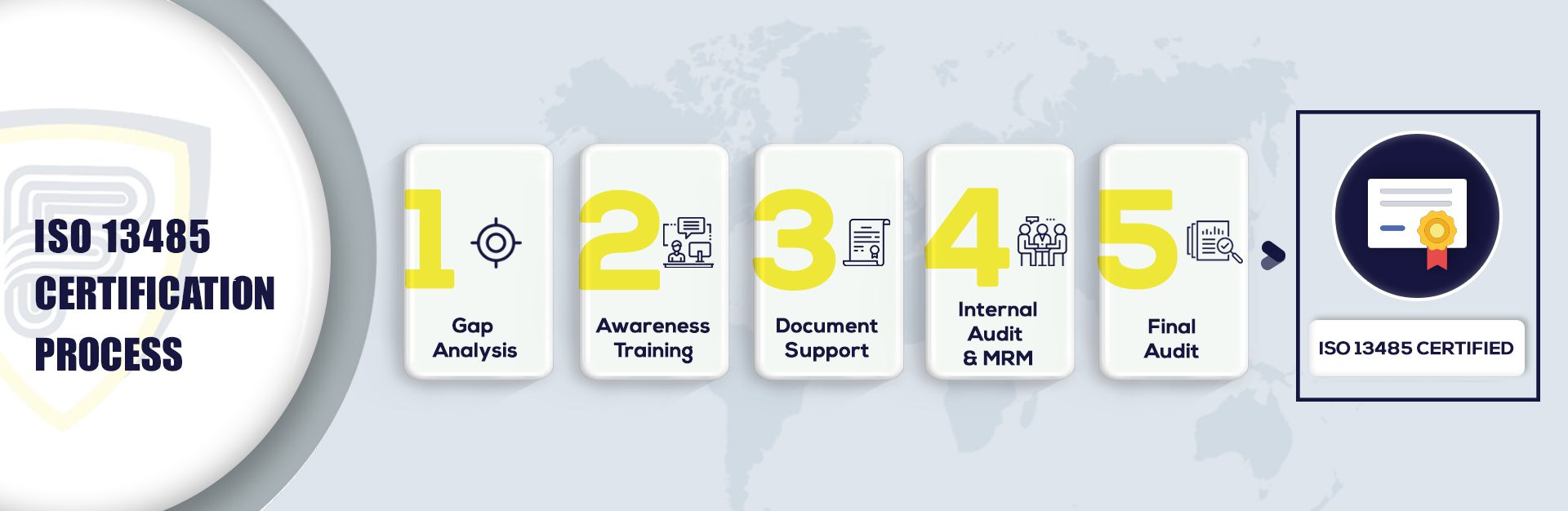

The ISO 13485 Consultant in Singapore provides ISO 13485 Certification services around Singapore, including Hougang, Tampines, Pasir Ris, Yishun, Choa Chu Kang, Bukit Batok, Queenstown, Clementi, Serangoon, etc. The services include implementation, documentation, auditing, process templates, training, gap analysis, and the registration process at an affordable cost for all organizations to become certified in pharmaceutical and medical manufacturing in Singapore.
This sector-specific variation on ISO 9000 ensures a satisfactory management system for medical devices under ISO 13485:2016 certification in Singapore. The company has a short history. With medical devices, you have to be very careful, and you should have a management system that guarantees the quality of the devices sent to the Marketplace. In addition to medical devices they self-include, components that go into the medical devices are becoming increasingly popular.
According to ISO 13485 Certification Services in Singapore, a documentation procedure is required for defining what is needed to review nonconformity, including customer complaints coma, which refers to the causes of dissent, which is also often called an analysis of the root cause, identifying actions to prevent recurrence to ensure nonconformity does not reoccur, defining how the movement must be determined and implemented, including updating the documentation, recording the results of investigations and actions taken, defining the documents, and reviewing the effectiveness of corrective actions taken.
When you register your ISO 13485 system in Singapore, you must identify the processes that apply to the quality management system and ensure that they are interconnected and controlled using a risk-based approach. You have to document your QMS to reference you do it in a quality manual. The device type or family must be confirmed in a medical device file. All documentation and records should be maintained as part of a documented procedure, and all forms should be kept where appropriate. ISO 13485 registration in Singapore requires commitment from the top management to maintain an effective quality management system. This means selecting the quality policy and setting the quality objectives for the whole organization.
To run the organization effectively, the top management must define and document the responsibilities and authorities of each department within the organization in conformity with its QMS. In Singapore, ISO 13485 audit services are available, including the appointment of a management representative who will prepare a report on the effectiveness of the QMS at scheduled management reviews. For the QMS to be effective, it is specified that the necessary resources be provided.
To ensure that competent people set the quality of the product, you must identify the people and the job descriptions. A medical device company provides the infrastructure and work environment necessary to ensure device safety and performance, such as health, cleanliness and clothing requirements. Where this could affect the product quality where applicable, you should ensure that you prevent contamination if the product meets this kind of control.
The consultant responsible for ISO 13485 certification in Singapore needs to ensure that the operational requirements for the accreditation begin with the planning and development of the processes required for ISO 13485 implementation in Singapore. You are supposed to set the quality objectives to meet the customer’s and regulatory requirements and make sure that there are necessary arrangements for communication with customers and medical device regulators. Consultants specializing in ISO 13485 Certification cost in Singapore should also organize design and development procedures.
Effective design verification and validation are very important requirements. The next step is to document the requirements for purchasing, such as determining which suppliers to select, monitoring their performance, and inspecting and verifying the vendor’s processes before the design is transferred to the manufacturer. You must plan and implement the production and service provision in compliance with ISO 13485. You are supposed to be fine. You can close for contamination to be controlled, which is applicable. Documentation of product installation and verification requirements is also required. You should also report the service in procedure, sterile medical devices and product process validation.
Are there any advantages to ISO 13485 certification in Singapore?
- Business expansion in international countries will increase profits for companies using ISO 13485 Consulting Services in Singapore.
- By obtaining ISO 13485 certification in Singapore, you will improve customer confidence in the product, which will lead to increased sales.
- Singapore ISO 13485 Consultants can help get more business from government tenders since participation is a mandatory requirement.
- All the risks involved in the process will be identified through ISO 13485 consultancy services in Singapore.
Why Choose Factocert?
A quality management system (QMS) is particular International standard is focused by the ISO 9000 family. ISO Certification Audit in Singapore states Quality management system has two significant pillars, one of which is the continual improvement, and the other is customer satisfaction. The organization must undergo many changes such as repairing quality policy, conducting meetings, recording minutes of the meeting, scheduling the meetings, documenting the reports, undergoing awareness training, preparations of SOP’s and many other significant changes will establish to bring a successful management system.
The implementation of a quality management system in one organization will emphasize product quality along with the service. The quality management system will assist the organization to retain the stability in the Marketplace and introduce the organization to have global trade. The implementation of a quality management system in the organization will establish seven quality management principles and many essential clauses which will bring the organization towards success. We provide ISO certification consultants in Singapore.
Medical device quality management system this particular International standard is focused by ISO 13485 standard. Know the best ISO certification bodies in Singapore. The quality management system mainly focuses on the establishment of a well-equipped medical device and customer service. This standard will help the organization to have an efficient workplace and successful implementation of medical equipment. ISO consultant services in Singapore will be certainly a beneficial factor.
An environmental management system is the second most world-widely recognized ISO standard. ISO consultants in Singapore states ISO 14000 families focuses on the environmental management that will help the organization to overcome all the damages that have been caused on the environment or minimize effects on the environment. The negative impact of the organization or any factor from the organization on climate can produce over land, air, or water. The environmental management system also focuses on the process improvement, which will attract the customer towards the organization.
ISO Certification in Singapore states the purpose of publishing this International standard is to make every organization eco-management friendly. Environmental management is the management of the adverse effects that are caused by human resource on nature. Here can be avoided or eliminated with the help of implementation of this environmental management system in the organizations. Many industries in the world of business will run through with the help of utilizing natural resources. Here will make a significant impact on the environment in the long run. The environmental management system will help the organization to identify such kind of threats and assist the organization to obtain sustainability for protecting the environment.
General requirements for the competence of testing and calibration Laboratories – the ISO 17025 focuses on the accuracy over the results of laboratories. ISO consulting services in Singapore helped in most of the countries testing and calibration standard and made compulsory to obtain mastery in the reports. Standard more specific in the requirement for competency concerning producing the testing and calibration results.
ISO 22000 family follows food safety management system. Food safety management system is known as FSMS in short. The international standard will help the organizations that are involved in any stage of the food chain to establish necessary procedures to protect the contamination of food. From storage till delivery of food to consumer, in any stage of preparation for storage, the disease can be possible. ISO audit service in Singapore is readily available.
Food system management system will establish strict rules and regulations to be followed to ensure the hazards on food eliminated in every stage of the food chains such as crop growing, storage of raw materials, transportation of raw materials, preparation of the food, packaging of the food and many other food-related activities. ISO certification consultants in Singapore helps all kind of industrial organizations which are involved in the food chain must ensure every safety should be provided to protect the contamination against the food. Factocert helps to find the best ISO certification process in Singapore.
It is the information security management system. An organization to establish a well-secured network concerning protecting the information flow inside the organization. ISO audit in Singapore assist with ISMS and will ensure the confidential data is available for the necessary individual and protected against all kind of threats from external sources. The integrity of acids and protected with the help of (information security management system integration) with the organization. The customer will have confidence for securing data which will increase integrity over the organization. ISO cost in Singapore is affordable.
Occupational health and safety management system this particular standard is related to workplace health and safety, which must be considered by the organization to provide a healthy environment for every employee in the company. The rule finds a multidisciplinary factor, which will concentrate on safety, health, and welfare of each employee who is working for the organization. The objective of this International Standard is to establish a safe and healthy work environment which will boost up the confidence of each individual in the organization.
ISO Certification in Singapore mainly helps the organization and also protect the workers, family members of each individual in the organization, employee customers, and many other factors which will be affected by the working environment. Some of the countries are made mandatory for the implementation of this ISO 45000 family which will focus on occupational health and safety management system in every organization. Get a free quote for ISO certification cost in Singapore.
ISO/IEC 20000-1: it’s information technology – service management system (SMS) standard. It defines requirements for your service supplier to program, determines, implements, operate, monitor, review, claims, and enhance the SMS. The prerequisites include the plan, transition, shipping, and development of services to meet agreed service demands.
ISO 31000, Risk management — Guidelines, supplies fundamentals, a frame, and a process for handling risk. It may be employed by any organization irrespective of its size, action, or business. ISO 31000 supplies a degree of reassurance concerning financial resilience, professional standing, and environmental and safety results.ISO 31000 is utilized throughout the life span of this organization and may be applied to virtually any action, such as decision-making in any way levels.
ISO 10002 standard is linked to Customer satisfaction and Supplies guidelines for complaints handling in organizations.This Standard provides guidance for the Practice of criticism Handling associated with goods in an organization, such as design, planning, performance, upkeep, and advancement. The complaints-handling procedure described is acceptable for use among the procedures of a general quality management system.
ISO 22301 is a Business continuity management system.This will verify the security of society out of and in reaction to, events, crises, and disasters brought on by intentional and accidental human actions, natural dangers, and technological failures.This standard specifies requirements to apply, preserve and enhance a management system to safeguard against, decrease the probability of the incidence of, and prepare for, react to and recover from disruptions if they appear.
ISO 50001 Made to encourage organizations in all industries, this ISO standard provides a sensible way to improve energy use, throughout the Growth of an energy management system (EnMS).This relies upon the management system model of persistent Advancement also employed for additional renowned standards like ISO 9001 or ISO 14001. This also makes it a lot much easier for organizations to incorporate energy management in their general efforts to increase quality and environmental management.
Explaining basic requirements for suppliers of learning services from non-formal education and training.ISO 29990 is also a quality management system standard for providers Of education and training services. ISO 29990 supplies a unified model for a quality and skilled manner of performance, in addition to a frequent reference point for both learning service providers (LSPs) and their customers for its conceptualization, development, and delivery of the designated program.
CE Mark the Conformity European (CE) Mark is described as the European Union’s (EU) mandatory conformity indicating for controlling the goods marketed in the European Economic Area (EEA) as 1985. The CE marking signifies a manufacturer’s statement that goods comply with the EU’s New Approach Directives. All these directives not just apply to goods inside the EU but in addition to goods that are made in or made to be marketed at the EEA. This produces the CE marking familiar worldwide even to people unfamiliar with the EEA.
There’s an EU demand that goods not in conformity with all the conditions of the directives aren’t permitted to circulate at the lands of the member countries.CE marking doesn’t provide any particular information to the consumer. It’s not a quality guarantee statement, but it doesn’t reveal proof of third-party testing, and it shouldn’t be mistaken with any certification mark of this kind issued by international or European informed examination bodies.
Particular directives include a choice for the Accountable organization to extend a statement of conformity saying a product meets the requirements of the related directives.
Halal is a phrase designating any item or an act that’s permissible to use or participate in, based on Islamic law. It’s the reverse of haram. The expression is employed to designate food viewed as permissible in accordance with Islamic law. Halal is the Arabic word meaning lawful or permitted. With regard to food, it’s the nutritional standard, as encouraged in the Qur’an (the Muslim scripture).
These conditions Are Generally Utilized in terms of Food goods, meat products, cosmetics, healthcare products, food, food ingredients, and food contact substances. When many items are obviously halal or haram, there are a number of things that aren’t very apparent. Additional information is necessary to categorize them as halal or haram.
HACCP Hazard analysis and critical control factors, or HACCP is a systematic preventative Strategy Into food safety by biological, compound, bodily dangers, and more lately radiological dangers in manufacturing processes which could result in the final product be dangerous and layouts steps to decrease the risks to a secure level. This way, HACCP tries to steer clear of hazards as opposed to trying to inspect finished goods for the consequences of these hazards.
The HACCP system May Be Used Whatsoever phases of a food series, from food manufacturing and preparation procedures such as packaging, supply, etc…HACCP was known internationally as a Logical instrument for adapting conventional review methods into a contemporary, science-based, food safety system. According to risk assessment, HACCP programs permit both businesses and authorities to devote their funds effectively by launching and auditing protected food manufacturing practices.
GMP: Good Manufacturing Practice (GMP) is a system for ensuring the products are consistently produced and controlled in accordance with quality standards. It’s intended to decrease the risks involved with any pharmaceutical manufacturing that must not be removed throughout testing the final item.
GLP: The OECD Principles of Good Laboratory Practice (GLP) guarantee the creation of high-quality and dependable test information linked to the safety of industrial chemical compounds and preparations.
The SA8000 Standard is the world’s top social certification program. Even the SA8000 Standard and Certification System supply a platform for organizations of all sorts, in almost virtually any market, and in almost any state to conduct business in a means that’s just and adequate for employees and also to show their adherence to their greatest social standards.
Made by SAI in 1997 as the first authoritative societal certification, it has caused the business for more than 20 decades.SA 8000 certification is a certifiable international standard that motivates an organization to employ, build and keep good social practices at work.
Vulnerability Assessment and Penetration Testing (VAPT) are equally security services that are devoted to identifying vulnerabilities in the system server, and system infrastructure. The two services serve another function and are completed to attain distinct but complementary objectives.
Capability Maturity Model Integration (CMMI) is a process-level advancement training and evaluation program. Administered by the CMMI Institute, it was developed at Carnegie Mellon University (CMU). It’s necessary for most U.S. Government contracts, particularly in computer program development.
CMMI is used to guide process development across a job, branch, or entire organization. CMMI defines the Subsequent maturity levels for procedures: First, Managed, Defined, Quantitatively Managed, and Optimizing.
Our Services
Our Clients










































ISO 13485 auditors in Singapore are essential because if you’re facing the ISO 13485 auditors in Singapore you have to be very careful about every parameter in your organization but when you join hands with Factocert. We as an ISO 13485 Consultants in Singapore, are tagged up with so many different ISO 13485 Certification bodies in Singapore .
Benefits of ISO 13485 Certification in Singapore
You will have the edge over your list of ISO 13485 certified companies in Singapore .
Marketing becomes very easy when you have certification tagged on to your name and which will help you to get into a global market and be a global player.
Employee satisfaction rates increases which are directly proportional to your employee retention and by retaining your critical employees you will have higher stability as a company
A government will recognize you for having such ISO 13485 Certification in Singapore
You will be automatically qualifying for any tenders because most of the companies require you to certify for ISO 13485 Certification in Singapore for participating in tender
Customer satisfaction rates will go high, and you will not have to face any more consequences from your customers or your vendors
Are you looking for
ISO 13485 Certification Consultants in Singapore
What else are you waiting for the only step you have to take care about is getting in touch with us, and we would take the best care, and in no time you would be certified for relevant ISO 13485 certification in Singapore you prefer. Most thing companies worry about is ISO 13485 service cost in Singapore but let us just tell you that ISO 13485 cost in Singapore is not what you should be thinking of because when this certification can give a boost to your organizations process.
We will make sure that the cost of ISO 13485 certification in Singapore is as minimal as possible. To get you ISO 13485 Certification in Singapore than we assure you 100% guarantee results and we ensure that you will definitely be certified because have 100% success rates till date in getting our customers certified. So get in touch with us as early as possible and get your ISO 13485 certification in Singapore at the earliest.
Mail us at contact@factocert.com for quick assistance.




















