ISO 13485 Certification In Iraq
ISO 13485 Certification in Iraq
ISO 13485 Certification In Iraq, Factocert is one of the top leading ISO 13485 Certification providers in Iraq. We provide the best ISO 13485 Consultant service in Baghdad, Mosul, Basra, Erbil, Najaf, Karbala, and other major cities in Iraq with service of implementation, training, auditing, and registration.
ISO 13485 Certification in Iraq is an industry-specific standard that is published by the international organization for standardization where ISO 13485 certification services in Basra specify the requirements of a quality management system where it focuses on the medical devices and related industries involved in the manufacturing process of medical devices.
ISO 13485 registration services in Erbil follows a set of requirements and other process interrelated which includes guidelines to implement, establish and maintain all the elements which are framed out to meet the standard customer requirements and their satisfaction and also by meeting a regulatory that required for the business in operating the medical device sectors. ISO 13485 registration in Baghdad is one best factor to adopt for the organization.
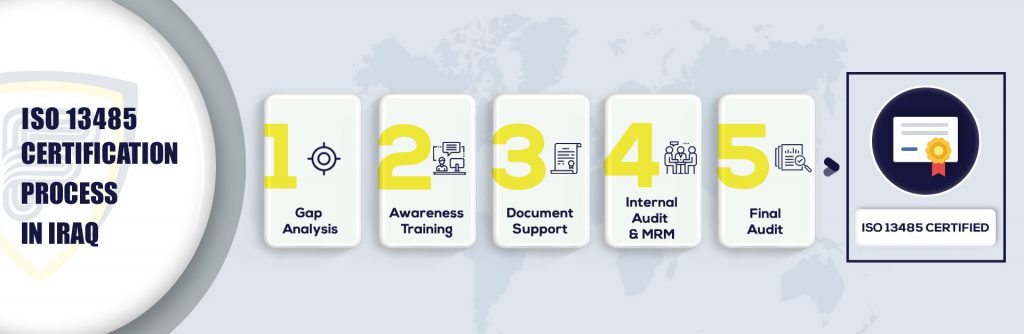
What are the steps to get ISO 13485 Certification in Iraq?

Requirements of ISO 13485 standard
To get certified by ISO 13485 audit services in Iraq for the medical device companies, they have to be approved by quality management system ISO 9001 which demonstrate the organization’s ability to provide services on the product related to medical devices which needs the regulatory and legal requirements along with the approval of the customers.
ISO 13485 certification consultant in Iraq helps to overcome the hazards, and it can apply to all the organization irrespective of size and location, but it focuses on the medical device manufacturing process and involves in one or more stages of medical devices life cycle
The life cycle includes development and design, manufacturing, production, packaging, storage, distribution, installation or any other services related to medical devices activities associated with supply chains, technical support or any other product-related services involved in consulting. ISO 13485 consultants in Iraq would help you on how to get ISO 13485 certification in Iraq and know more visit our website www.factocert.com
And there are no separate testing requirements for ISO 13485 standard, but there is the standard which helps to meet those requirements. As discussed earlier ISO 13485 Certification in Iraq, which is specific to management requirements, and it is considered to be standalone. The medical device manufacturers or any other service organization should provide evidence in documentation format that their devices are meeting the quality and safety requirements and it performs according to the intended which are suitable for the end-users patients are any consumers. So coming to the testing, it is a part of the quality management system that helps to implement the requirements of the standard and along with the various regulations. ISO 13485 certification cost in Iraq is beneficial.
Why medical manufacturing industries should have a quality management system?
One of the long-term strategic decisions is implementing a quality management system because it helps you to identify all the risks and hazards and guides your organization to meet the requirement of the standard consistently by improving the overall performance. ISO 13485 audit in Iraq also helps to how are sustainable development and the quality management system gives an assurance to the customers that is meeting all the requirements of the quality system apps to build confidence for the manufacturers and also the customers. Now recently the news has come from the FDA of US that they also will use ISO 13485 standards as a foundation for the quality system solution for the medical devices.
We know ISO 13485 audit service in Iraq is one of the International standards that specify the requirement of quality management system for medical devices which helps to meet all the needs along with the legal requirements. Thirteen thousand four hundred eighty-five standards provide a framework very effectively so that the work is done effectively and transparently in the process. It also helps to gain the recognition of the organization that it has met the requirements of the standard. ISO 13485 cost in Iraq and to know more contact us at contact@factocert.com.
Transition facts of ISO 13485 certification in Iraq
- ISO 13485 certification process in Iraq focuses on the life cycle of medical devices includes development design storage distribution production installation decommissioning and servicing. To meet the regulatory requirements, the standard designed in such a way that they focus on their management system along with the risk management approach.
- Now let us go through the transition factors of the ISO 13485 standard:
- ISO 13485:2003 and ISO 13485:2012 is replaced by ISO 13485:2016. published in the year 2016 on 1st March
- ISO 13485 standards are aligned with ISO 9001 2008 but not with ISO 9001 2015. This misalignment did due to the revision of the rules with one another.
- The technical ISO committee introduced these changes and managed them. It is the role of the technical committee to decide upon the revision of the standard, and it was done to ensure the quality management system can be integrated for development for specific products and requirements which outlined for the directives for particular devices such as:
- Vitro Diagnostic medical device directive (IVDD) 98/79/EC & Medical Directive (MDD) 93/42/EEC.
- Due to the increased competition in the market, technical transition, and complexity of organizations, the concept of revision came into existence.
Specification of ISO 13485 standard
- Develop and identify the quality management system for medical devices
- For the medical devices utilizes
- The control process and quality systems
- For the medical devices maintain and monitor the quality management system
- ISO 13485 standards requirements are specific to the industries devices of medical respective of type or size of the organization.


Every industry has its following scope, so if the regulatory requirements exclude the development and design controls used as a proof for the exclusion standard ISO 9001 quality management system.
The alternative arrangements by the regulations and it are the duty and responsibility of the organization to meet all the conformities of ISO 13485 certification bodies in Iraq excluding the development and design controls.
The top management and the organization should take responsibility if the process that is required by ISO 13485 consulting services in Iraq applies to medical devices and is not performed by the expert or in charge of the organization.
Our services:
ISO 9001 Certification
ISO 9001 sets out the criteria for a quality management system. It may be used by any organization, big or small, irrespective of its field of activity. There are more than one million businesses and organizations in over 170 countries certified to ISO 9001.
ISO 14001 Certification
ISO 14001 Sets out the standards for an environmental management system and can be certified to. It maps a frame that a business or organization can follow to set up a successful environmental management system.
ISO 14001 helps an organization achieve the planned results of its environmental management system, which provides value for the surroundings, the organization itself, and interested parties.
ISO 17025 Certification
ISO/IEC 17025 enables laboratories to show that they function competently and generate valid outcomes, thus fostering confidence in their work both universally and across the globe.
Additionally, it helps ease cooperation between laboratories and other bodies by producing broader acceptance of effects between states. Evaluation reports and certificates may be accepted from 1 nation to another without the need for further testing, which, in turn, improves international trade.
ISO 22000 Certification
ISO 22000 lays out the prerequisites for a food safety management system. It maps out what an organization has to do to demonstrate its ability to control food safety hazards to make certain food is safe. It may be used by any organization regardless of its size or place from the food chain.
ISO 27001 Certification
ISO/IEC 27001 standard Are the employment of joint ISO and IEC (International Electrotechnical Commission) the technical committee, Information security, cybersecurity, and private security.
ISO/IEC 27001 is widely understood, providing prerequisites for the information security management system (ISMS), although there are over several dozen standards in the ISO/IEC 27000 family. Applying them empowers organizations of any sort to deal with the security of resources like financial information, intellectual property, employee information, or information secured by third parties.
ISO 45001 Certification
ISO 45001 specifies requirements for the Occupational health and safety (OH&S) management system, and provides advice for its use, to allow organizations to supply safe and healthy workplaces by preventing work-related injury and ill health, as well as by improving its OH&S performance.
ISO 20000-1 Certification
it is an information technology – service management system (SMS) standard. It defines requirements for the service supplier to program, determines, implements, operate, monitor, review, claims, and improve an SMS. The requirements include the plan, transition, shipping, and enhancement of services to meet agreed service conditions.
ISO 31000 Certification
Risk management — Guidelines, provides fundamentals, a framework, and a process for managing risk. It may be used by any organization no matter its size, action, or sector. ISO 31000 supplies a level of reassurance concerning economic resilience, professional reputation, and environmental and safety results.
ISO 10002 Certification
ISO 10002 standard is related to Customer satisfaction and Supplies guidelines for complaints handling in organizations
This Standard guides the process of complaint Handling associated with products in an organization, such as design, planning, operation, upkeep, and advancement. The complaints-handling process described is acceptable for use as one of the procedures of a general quality management system.
ISO 22301 Certification
ISO 22301 is a Business continuity management systems This will verify the security of society out of, and in reaction to, events, emergencies, and disasters caused by intentional and accidental human actions, natural dangers, and technological failures.
ISO 50001 Certification
ISO 50001 Made to support organizations in all industries, this ISO standard provides a practical way to improve energy use, during the Growth of an energy management system (EnMS).
ISO 29990 Certification
specifies basic requirements for providers of learning services within non-formal education and training.
CE Mark
CE Mark The Conformitè Europëenne (CE) Mark is defined as the European Union’s (EU) mandatory conformity indicating for regulating the goods sold in the European Economic Area (EEA) since 1985.
The CE marking represents a manufacturer’s statement that products comply with the EU’s New Approach Directives. These directives not only apply to products within the EU but also to goods that are made in or intended to be sold at the EEA. This produces the CE marking recognizable worldwide even to those unfamiliar with the EEA.
Halal Certification
Halal is a word designating any item or an action that’s permissible to use or participate in, according to Islamic law. It’s the reverse of haram. The term is used to designate food seen as permissible according to Islamic law. Halal is an Arabic word meaning lawful or permitted. Regarding food, it’s the nutritional standard, as prescribed in the Qur’an (the Muslim scripture).
HACCP Certification
HACCP Hazard analysis and critical control factors, or HACCP is a systematic preventive approach to food safety by biological, compound, bodily dangers, and more lately radiological risks in manufacturing processes which may lead to the final product be unsafe and designs measures to decrease these risks to a secure level. This way, HACCP attempts to steer clear of hazards as opposed to attempting to inspect finished goods for the effects of these hazards.
GMP Certification
Good Manufacturing Practice (GMP) is a system for ensuring that products are consistently produced and controlled following quality standards. It is intended to minimize the risks involved with any pharmaceutical manufacturing that may not be removed through testing the final product.
GLP Certification
The OECD Principles of Good Laboratory Practice (GLP) guarantee the creation of high-quality and dependable test data linked to the safety of industrial chemical substances and preparations.
SA 8000
The SA8000 Standard is the world’s major social certification program. Even the SA8000 Standard and Certification System provides a framework for organizations of all types, in almost virtually any business, and in almost any state to conduct business in a way that is fair and decent for employees and also to demonstrate their adherence to the highest social standards. Created by SAI in 1997 as the first credible social certification, it has caused the business for more than 20 years.
VAPT Certification
Vulnerability Assessment and Penetration Testing (VAPT) are both security services that are devoted to identifying vulnerabilities in the system server and system infrastructure. Both the services serve a different purpose and are carried out to achieve different but complementary targets.
CMMI Certification
Capability Maturity Model Integration (CMMI) is a process-level advancement training and evaluation program. Administered by the CMMI Institute, it was developed at Carnegie Mellon University (CMU). It’s needed by most U.S. Government contracts, especially in software development.
CMMI is used to guide process development across a job, branch, or entire organization. CMMI defines the Subsequent maturity levels for processes: Initial, Managed, Defined, Quantitatively Managed, and Optimizing.
Importance of ISO 13485 Certification in Iraq
- ISO 13485 consultancy in Iraq is beneficial in many ways if the organization is going to adopt the ISO 13485 Certification in Iraq.
- ISO 13485 consultant services in Iraq helps to meet the regulatory requirements in easy go for medical devices manufacturers on international trade.
- ISO 13485 specifies a condition of the quality management system and helps the organization to develop service, produce and design the medical devices.
- By getting certified by ISO 13485 Certification in Iraq will help to achieve the benefits of suppliers, customers, workers and also the management.
- Helps in improvement continuous assessment and re-registration of the system.
- ISO 13485 consultancy services in Iraq in walls management approach consist of assessments towards the identification and estimation of the risk, control, and techniques associated with the risk eliminate all situations which are hazardous and might cause accidents in the future throughout the entire process of product realization.
- ISO 13485 certification consultants in Iraq act as one of the assurances to export the products to the Global markets, which help in the increase of profits.
- It gives warranty to customers and also to the end-users that the product is manufactured based on the requirements of 13485 standards.
- To get out and out from the government sectors, the organization has to be certified by ISO as it things approved by the measure, would have made all the requirements and followed all the quality systems which and sure to provide the best effective product at the end.
- By implementing ISO 13485 Certification in Iraq helps to build a connection between the employees having a sound communication system in the organization and allows all the employees to take the initiative and helps to create a leadership quality as well. These are the few benefits of implementing the ISO 13485 standard to your industries which would be helpful in many ways. For more information please visit ISO 13485 Certification in Iraq
Our Services
Our Clients










































ISO 13485 audit services in Iraq are essential because if you’re facing the ISO 13485 audit in Iraq you have to be very careful about every parameter in your organization but when you join hands with Factocert. We as an ISO 13485 Consultancy Service provider in Iraq, are tagged up with so many different ISO 13485 certification bodies in Iraq.
Benefits of ISO 13485 Certification in Iraq
You will have the edge over your list of ISO 13485 certified companies in Iraq.
Marketing becomes very easy when you have certification tagged on to your name and which will help you to get into a global market and be a global player.
Employee satisfaction rates increases which are directly proportional to your employee retention and by retaining your critical employees you will have higher stability as a company
A government will recognize you for having such ISO 13485 Certification in Iraq
You will be automatically qualifying for any tenders because most of the companies require you to certify for ISO 13485 Certification in Iraq for participating in tender
Customer satisfaction rates will go high, and you will not have to face any more consequences from your customers or your vendors
Are you looking for
ISO 13485 Certification Consultants in Iraq
What else are you waiting for the only step you have to take care about is getting in touch with us, and we would take the best care, and in no time you would be certified for relevant ISO 13485 Certification in Iraq you prefer. Most thing companies worry about is ISO 13485 service cost in Iraq but let us just tell you that ISO 13485 cost in Iraq is not what you should be thinking of because when this certification can give a boost to your organization’s process.
We will make sure that the cost of ISO 13485 Certification in Iraq is as minimal as possible. To 13485 get you ISO Certification Services in Iraq then we assure you 100% guarantee results and we ensure that you will definitely be certified because have 100% success rates to date in getting our customers certified. So get in touch with us as early as possible and get your ISO 13485 Certificate in Iraq at the earliest.
Mail us at contact@factocert.com for quick assistance.




















