GMP Certification In Jordan
GMP Certification In Jordan
GMP Certification in Jordan and its cities like Amman, Zarqa, Irbid, and Russeifa with implementation, training, documentation, gap analysis, registration, audit, to all organizations seeking certification under the Good Manufacturing Practice management system in Jordan.
One of the best practices establishing the criteria for good manufacturing practices to be adopted inside the firm process, leading to greater process efficiency and improvement, is GMP certification in Jordan, which is one of the internationally recognized standards.This particular standard, which is relevant to the food and pharmaceutical industries, is mainly concerned with management systems, document control, cleanliness, and hygiene.
GMP certification services in Amman are a result of many management methods. It is a well-known process certification that is offered in the industry. GMP, or good manufacturing practice, is a system that makes sure that goods regularly meet quality requirements. The Food and Drug Administration (FDA) established GMP requirements to reduce the hazards associated with manufacturing products like supplements and other medications.
GMP encompasses all facets of production, including the tools, locations, and materials as well as the staff’s PPE and personal hygiene. GMP rules state that any step that can have an impact on the quality of the end product must have specifically defined procedures.To demonstrate that every product is created consistently, every stage of the manufacturing process must be supported by written documentation of the techniques utilized.
What Are The Steps To Get GMP Certification In Jordan?
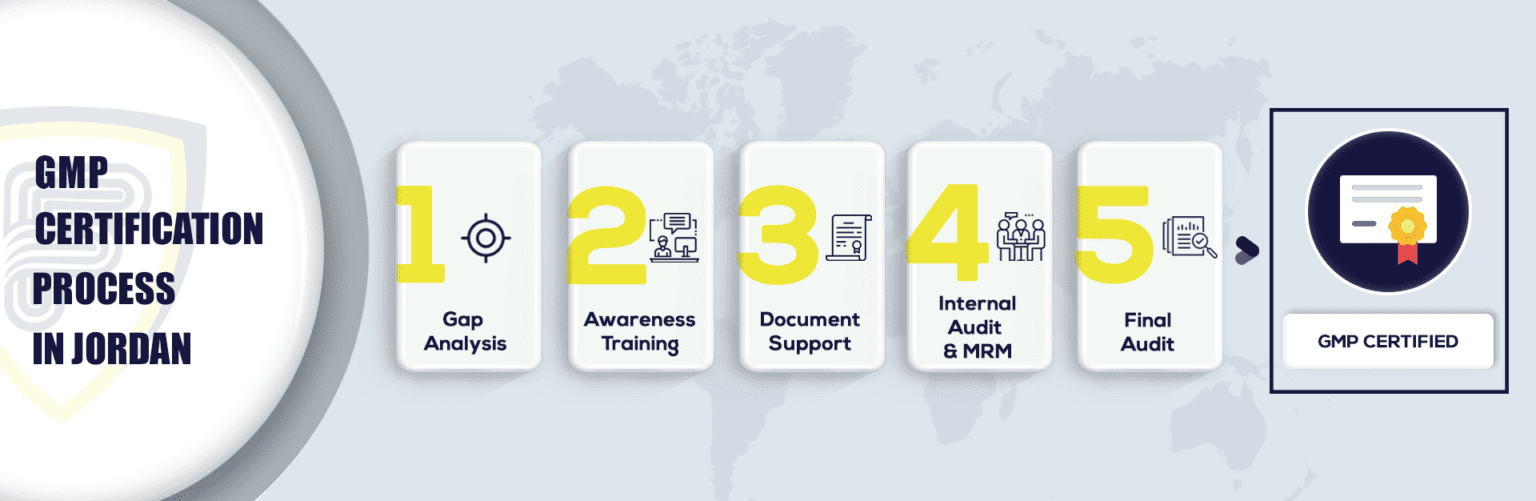

Why is GMP Certification in Jordan important?
It is crucial to have a certified GMP producer. By merely glancing at the pill, it is practically hard for a consumer to know whether a medicine is safe or effective. GMPs are essential because they shield consumers from potentially dangerous supplements and medications.
GMP Certification not only demonstrates that manufacturers create their goods to the greatest degree of quality possible, but it also offers them a solid reputation as a reliable and responsible facility. A producer may ensure that a product was produced in an FDA-approved facility if they have a GMP Certification. Additionally, this implies that the producer is carrying out all required quality control procedures to create items that are reliable and efficient.
Assurance of the food’s safety and quality is a key factor for customers nowadays. A GMP is a crucial addition to your food safety management system that raises consumer trust in your dedication to trading and manufacturing high-quality, safe foods.
All food processing facilities must adhere to basic, hygienic and processing criteria to comply with GMP regulations. To develop and execute additional quality and food safety management systems, such as HACCP, ISO 22000, SQF, and ISO 9001, many organizations in the food sector first adopted the GMP Certification scheme for food processing.
The fundamental manufacturing procedures and requirements required for the execution of a successful Hazard Analysis Critical Control Point (HACCP) food safety program are independently verified and certified by a Good Manufacturing Practices (GMP) certification system.
In terms of independent certification and verification, Factocert is a global leader. Having your food safety management system certified by Factocert following GMP standards is a great approach to be ready for inspections by regulatory bodies and other stakeholders. While showing your understanding of the significance of producing and selling safe, high-quality food, the method will assist you in ensuring regulatory compliance.
GMP registration services in Irbid may aid in streamlining the production process and creating an efficient management structure. This offers instructions on how to get your process up to the level of all internationally successful businesses.
GMP certification in Zarqa is essential for minimizing the waste that will be produced throughout the production process. There is a huge demand for the standard since more manufacturing enterprises have recently established themselves here. To compete with your competitors, it is crucial to get the certification or any international standard that is relevant to your industrial area.
A GMP Certification consultant in Jordan may make a significant contribution to your organization’s adoption of a new management system that improves the efficiency of your business processes. One of the main issues with not engaging a consultant is that the process heads will get locked in and their costs will exceed the budgeted amount.
A GMP consultant in Amman who has the necessary expertise and comprehension of the industry can help the business incorporate best practices into its operations. If you want to double-check your management system, it is best to use GMP audit services in Irbid, which may help you find flaws at the micro level of your business. Your business may compete with the finest market leader in your industrial area by using GMP in Zarqa.
What benefits does GMP Certification offer?
- GMP consulting services in Jordan will assist in increasing the value of your brand to the point where your sales graph begins to improve.
- GMP Consulting in Amman can assist you in obtaining orders from the public and government sectors.
- GMP consulting in Irbid may assist you in reducing unnecessary marketing costs, and increasing gross margin and profit.
- GMP consultation in Zarqa may help your process become more effective.
How Can One Get GMP Certified In Jordan?
In terms of your certification needs, we are one of the one-stop solution providers. We are a reputable consulting firm that is known all over the globe. We guarantee that your certification requirements have been fully satisfied at a fair price.
We put a greater emphasis on assisting our clients in getting the most out of the certification and the standard. During the external audit, our expert will assist you in providing proof of the management system that has been put in place to meet the standard requirement. Regardless of business size or annual revenue, it is always clear that with Factocert, GMP Certification cost in Jordan is consistently low and affordable for all of our clients.
A successful manufacturing process may be implemented with the support of GMP standards, which are a set of principles that guarantee quality is ingrained throughout the business and the procedures involved. GMP regulations are typically forgiving, and each nation has its laws that must adhere to its own GMP principles and regulations. However, practically all rules are drawn from the fundamental idea and principles that are:
- Quality Control:
The goal of quality management is to guarantee that produced goods are suitable for their intended use, conform to specifications, and do not endanger customers via insufficient safety, quality, or effectiveness controls. Quality assurance, excellent manufacturing processes, quality control, and quality risk management should be fully and accurately executed to meet this quality aim.
- Quality Management:
The goal of the quality assurance system is to make sure that produced goods are conceived and designed in a manner that complies with GMP standards.
- Guidelines for Good Manufacturing Practice:
Good manufacturing practice is concerned with production and quality control as a component of quality assurance. It seeks to lessen the production-related hazards that are unavoidable. The following are its fundamental needs as stated by WHO’s Good Manufacturing Practices for Pharmaceuticals:
- All manufacturing procedures are precisely specified, routinely examined in light of experience, and shown to be capable of producing consistently high-quality pharmaceuticals that adhere to their specifications and/or marketing permission.
- Validation of crucial manufacturing process stages and substantial process modifications.
- All GMP-related facilities, such as:
- Employees who are suitably qualified and trained;
- Adequate Premises;
- Appropriate Tools and Services;
- Right supplies, Packaging, and Labelling
- Authorized Practices and Guidelines;
- Procedures Written in an instructive format using straightforward language that is directly pertinent to the facilities offered, instructions and procedures;
- Operators get the proper training to follow protocols;
- During production, records are kept manually or with the use of recording devices to show that all the processes outlined in the procedures and instructions were followed and that the product’s quantity and quality were as anticipated. Any notable differences are thoroughly noted and looked into;
- Manufacturing and distribution records that allow for the whole history of a batch to be tracked are kept in a clear and understandable format;
- The items’ quality is less at risk due to distribution (wholesaling);
- There is a procedure in place to stop the sale or supply of any batch of a product;
- Product complaints are investigated, the sources of quality flaws are looked into, and suitable action is made to address the faulty items and avoid recurrence.
- Quality Assurance:
Good Manufacturing Practice has a component called quality control that emphasizes sampling, specification, and testing. To make sure that goods pass the necessary tests before being launched for sale or supply, it examines the setup, documentation, and release processes.
- Effective Risk Management:
Assessment of risks that may have an impact on product quality is done systematically via the process of quality risk management. Its guiding principles include that good risk management should make sure of the following:
- The assessment of the quality risk is based on scientific expertise, and process expertise, and, ultimately, connects to the protection of the patient and users;
- The effort, formality, and paperwork put into the quality risk management process correspond to the risk’s magnitude.
- Cleanliness and Hygiene:
In every step of the production process, sanitation and hygiene are essential. It includes everything that might potentially contaminate a product, including workers, the environment, tools, containers, and manufacturing supplies. A thorough sanitation and hygiene program should be used to identify and eradicate any possible sources of infection.
- Structure and Surrounding Properties:
As a concept, the premises should be positioned in an environment that is suited for its activities and one that is free from hazards of contamination of materials and products. Additionally, the location should be simple to keep clean and maintain and should be built to reduce operational mistakes.
- Equipment:
Similar to the premises, equipment should be positioned, maintained, and designed to work for its intended purpose. It should also be cleaned and preserved following protocols. It should be removed or marked as faulty if there is a flaw or problem.
- Raw Materials:
All production-related materials must be kept appropriately following the guidelines established by the manufacturers. To guarantee that all incoming materials are accurate and of good quality, a suitable stock management system should be put in place.
- Personnel:
The individuals carrying out GMP compliance are crucial to its success. All employees must be trained and competent to do their jobs for this reason. They should be educated on the GMP principles and given ongoing training, and other resources suited to their requirements. Compliant supervisors should be explicit about each employee’s job descriptions to prevent misunderstandings and lower the possibility of problems like overlapping roles.
- Qualification and Attestation:
Validate if processes and procedures can consistently generate high-quality goods by evaluating whether systems, facilities, and equipment are suitable for and ready for the application for which they are intended. Verification of crucial production procedures is necessary to guarantee that high standards of product quality are consistently met.
- Complaints:
GMP includes handling complaints, thus every manufacturing company should have a GMP complaint procedure that is properly thought out. Ideal complaint management should have a prepared answer to cover all eventualities.
- Record-keeping and Documentation:
The quality assurance system must include effective documentation and record keeping to comply with GMP regulations. Managers and supervisors may maintain track of the historical record of production methods and corrective actions taken with the use of accurate recordkeeping. The following are the standards for documentation generally:
- Care must be taken in the design, preparation, review, and distribution of documents.
- Written materials should be readable and clear.
- Appropriate and authorized employees must approve, sign, and date documents.
- The substance of documents, such as their title, nature, and purpose, must be clear.
- Documents need to be updated and checked often.
- Documents cannot be written by hand.
- Any changes to a document or record must be initialled, signed, and dated. Recording the correction’s justification is also necessary (where appropriate).
- Keep a record of every step you perform throughout traceable processes like product manufacture and control.
- Examinations and Quality Checks:
Inspections should be carried out often to check on the application and observance of GMP. Record the areas that need more effort and provide remedial actions for ongoing progress.
Quality audits are performed to evaluate the manufacturing company’s quality systems. Companies may follow GMP regulations established by regulatory agencies with the use of GMP audit checklists. You may find non-compliant processes and act right once to address areas for improvement by carrying out site visual walkthroughs and conducting manufacturer reviews.
How to Comply With the Guidelines of the GMP Standard?
GMP standards and guidelines include a variety of topics that might affect a product’s safety and quality. The firm benefits from adhering to GMP or cGMP standards by increasing the quality of its goods, improving customer satisfaction, increasing revenues, and realizing a lucrative return on investment.
Assessing an organization’s compliance with manufacturing methods and requirements includes doing GMP audits. Regular inspections may reduce the possibility of adulteration and misbrand. The following systems, among others, benefit from an overall improvement in performance brought about by a GMP audit:
- Facilities and a building
- Material Control
- Quality assurance procedures
- Manufacturing
- Identification labels and packaging
- Quality control procedures
- GMP and personnel training
- Purchasing
- Customer support
GMP, or good manufacturing practices, guarantee that items are manufactured in a manner that satisfies quality requirements while also controlling quantity.
GMP takes into account several variables, including cleanliness and environmental conditions. Any faults or abnormalities may be found sooner via this method than they would be after the finished product has been evaluated or is on the market. Ensuring that your goods follow GMP will result in high-quality, safe-for-use or consumption finished products, which will improve customer satisfaction and generate more income.
What criteria must a company meet to be certified under GMP in Jordan?
Once a manufacturer has GMP certification in Jordan, it is its primary obligation to adhere to all the guidelines outlined in the standard to make goods that are entirely safe and sanitary. Typical GMP certification criteria in Jordan include the following:
- The production process must be preserved in writing, and records must be carefully maintained.
- The production space should be kept as clean as possible.
- All forms of impurities should be kept out of the production area, and the process should start in a controlled, sanitary atmosphere.
- Any changes to the production process must be confirmed right away to stop the development of any form of fault or defect.
- Written records of each staff member’s training and experience should be kept.
- To ensure a smooth and effective work environment within the production unit, a grievance box must be kept on the company’s property where employee complaints may be filed and promptly resolved.
- To maintain the highest degree of cleanliness in the workplace, employees and staff must get the appropriate training.
- Employees in management information system departments need sufficient training to keep accurate written records.
A company’s ability to increase client trust that the product they are purchasing is entirely sanitary and devoid of any form of pollutants is aided by receiving GMP certification in Jordan. This immediately aids manufacturers in increasing profits. For more information, you can reach our specialist group from our website:www.factocert.com or get in touch with our team by writing to us at contact@factocert.com and get the answer to all your inquiries.
Why choose Factocert for GMP Certification in Jordan?
Factocert can assist you if you want to learn more about the GMP certification process in Jordan. Our specialists will assist you in obtaining the finest GMP Certification in Jordan at reasonable costs, as well as a certification procedure that runs smoothly.
For more information Visit: GMP Certification in Jordan
Our Services
Our Clients










































GMP audit services in Jordan are essential because if you’re facing the GMP audit in Jordan you have to be very careful about every parameter in your organization but when you join hands with Factocert. We as a GMP Consultancy Service provider in Jordan, are tagged up with so many different GMP certification bodies in Jordan.
Benefits of GMP Certification in Jordan
You will have the edge over your list of GMP certified companies in Jordan.
Marketing becomes very easy when you have certification tagged on to your name and which will help you to get into a global market and be a global player.
Employee satisfaction rates increases which are directly proportional to your employee retention and by retaining your critical employees you will have higher stability as a company
A government will recognize you for having such GMP Certification in Jordan
You will be automatically qualifying for any tenders because most of the companies require you to certify for GMP Certification in Jordan for participating in tender
Customer satisfaction rates will go high, and you will not have to face any more consequences from your customers or your vendors
Are you looking for
GMP Certification Consultants in Jordan
What else are you waiting for the only step you have to take care about is getting in touch with us, and we would take the best care, and in no time you would be certified for relevant GMP certification in Jordan you prefer. Most thing companies worry about is GMP service cost in Jordan but let us just tell you that GMP cost in Jordan is not what you should be thinking of because when this certification can give a boost to your organizations process. We will make sure that the cost of GMP in Jordan is as minimal as possible.
To get you GMP Certification Services in Jordan than we assure you 100% guarantee results and we ensure that you will definitely be certified because have 100% success rates till date in getting our customers certified. So get in touch with us as early as possible and get your GMP certificate in Jordan at the earliest.
Mail us at contact@factocert.com for quick assistance.
Frequently Asked Questions
What is GMP Certification in Jordan?
The GMP Certification in Jordan stands for International Organization for Standardization. It plays an essential role in maintaining various market sectors’ standards. It starts right from manufacturing an item to providing a product. It is an independent, international organization that develops standards for ensuring the safety, quality, and efficiency of the services and products across Jordan and cities like, Amman, Zarqa, Irbid, Russeifa.
Who Needs GMP Certification in Jordan?
For industries in Jordan, GMP certification might be called for by legislation or contractually. But, even if that’s not the situation, satisfying GMP criteria has many advantages for organizations: Saving money and time by recognizing and resolving persisting issues, Improving system, and process effectiveness.
What are the types of GMP Certifications mandatory in Jordan?
While all the Standards are necessary for different organizations, some of the mandatory Certification Standards in Jordan are:
- GDP Certification: Good Distribution Practices
- GLP Certification: Good laboratory practice
- GMP Certification: Good Manufacturing Practices
- GDPR Certification: General Data Protection Regulation
- SOC 1 Certification: System and Organization Controls 1
- SOC 2 Certification: System and Organization Controls 2
- SA 8000 Certification: Social Accountability
- RoHS Certification: Restriction of Hazardous Substances
What is the cost of GMP Certification In Jordan?
Although the cost of GMP Certification in Jordan depends on the type of GMP Standards, Factocert provides the best GMP Certification services at the most affordable price across Jordan.




















