GMP Certification In South Africa
GMP Certification in South Africa
GMP Certification in South Africa,Factocert is the most well-known GMP Consultant in South Africa, offering GMP Certification in South Africa, Cape Town, Johannesburg, Durban, Pretoria, Bloemfontein, Port Elizabeth, Soweto, Germiston East London, Polokwane, and Pietermaritzburg with the services of Implementation, Documentation, Audit, gap analysis, training, and Registration process to all Good manufacturing Practice Certification in South Africa industries at a reasonable price.
The most widely used certification standard in South Africa is the GMP certification, which is adopted by practically all organisations because to its usefulness and significance. South Africa, also known as the Republic of South Africa, is a country in southern Africa. A diverse range of cultures, languages, and faiths are present in South Africa’s multiethnic community.
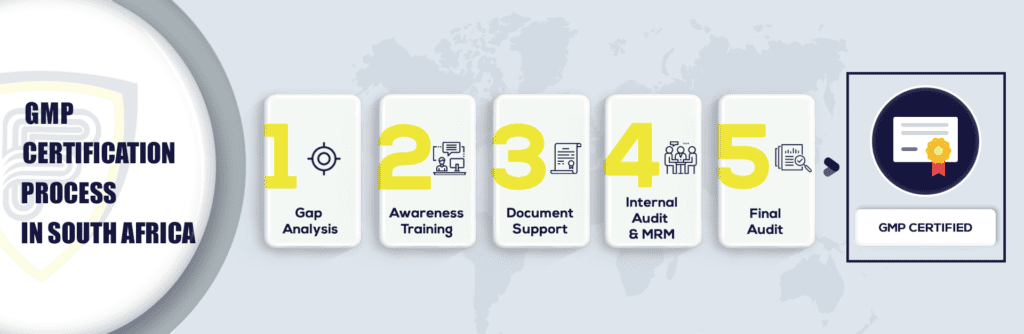
What are the steps to get GMP Certification in South Africa?

Economy of the nation is diverse. South Africa is a fantastic travel destination, and the nation’s tourism industry is a reliable source of income. South Africa is being helped to develop by many other large nations, and investors are arriving to assist the nation’s free market get started.
The nation began operations by establishing industries and businesses with the goal of halting brain drain. Any firm in South Africa that wants to achieve continuous improvement and customer satisfaction must now adapt to GMP.
GMP Certification in South Africa: A Reliable Method!
Good Manufacturing Practices certification in Cape Town.
The regulatory organisations that manage the licencing of food and drug manufacturing and distribution publish the GMP guidelines. The strategy can be more effectively used by a GMP certification specialist in South Africa.
The GMP Certification in South Africa guidelines outline the fundamental procedures that a manufacturer of pharmaceutical or food products must follow to guarantee that the goods being produced are of an acceptable calibre and are secure for use by the general public. You can receive thorough assistance from a GMP specialist in South Africa.
Current Good Manufacturing Practices, or GMP, is a common abbreviation for GMP. The organisation may be required to abide by the GMP Certification in South Africa guidelines in certain instances, but in many others, it will be necessary for the organisation to create and implement its own quality control procedures to meet GMP requirements.
South Africa’s GMP consultant service is the ideal remedy. GMP is a formal methodology that all pharmaceutical and food manufacturing sectors across the world must adhere to. The GMP certification audit in Cape Town will guarantee the purity of the product, ensuring that it does not hurt consumers or the general public, as well as the safety and quality of food and pharmaceutical items without any contamination.
South Africa’s GMP consulting service is a useful source. Signage on the containers is also crucial so that the wrong medications are not given to the patient. As a result, a through return procedure in the form of formal documentation is a fundamental necessity and demand of GMP to avoid any kind of manual error given the importance and importance of food and pharmaceutical product safety.
GMP Certification Guidelines in South Africa:
To ensure that food of pharmaceutical on medicine product meets the safety and healthy level for consumer consumption, GMP Certification in South Africa guidelines are a guide for manufacturing testing and quality assurance. In Cape Town, the GMP audit will outline all the rules. It involves much more than just enforcing numerous strict regulations on an organisation to ensure proper GMP.
Guidelines to get GMP Certification in South Africa.
- It is crucial to maintain a clean and sanitary working environment.
- Environmental factors should be under control to avoid adult-related contamination of pharmaceutical and food products that could be harmful for human consumption.
- To ensure that there are no deviations from the normal method, it is important to outline the manufacturing process and procedure in detail and submit proper documentation. It is highly advised to use the Factocert GMP consultant service.
- The procedure and process are managed, and any necessary adjustments are assessed. Modifications that impact the drug’s quality are acknowledged as required
- The methods, procedures, and instructions are described in simple language that is understandable to the average person.
- To prevent incorrect medication from reaching the end user, containers are kept with the appropriate signage. It is worthwhile to invest in GMP certification in South Africa.
- To ensure that the same deviation does not occur again, all faults or errors that were made during the process are identified and documented in a formal document.
- The quality of the finished product shouldn’t have an impact on how it is distributed.
- There is a method accessible for dialling any supply selling branch. Your business will be accredited with the aid of GMP certification companies in Durban.
- Any complaints about devices for products that are already on the market are looked into for quality issues, and then the necessary corrective measures are implemented to fix any problems and stop the same mistake from occurring again.
These GMP guidelines must be adhered to by the food and pharmaceutical industries in order to prevent product contamination at any point during the product’s life cycle of manufacture. In South Africa, GMP costs are one-time expenses. These instructions describe the actions to be taken in order for the Center process to be operated in a safe manner. Many large organisations that are moving in the right direction employ the method because it can’t just be said that GMP has benefits.
What is the procedure to get GMP Certification in South Africa?
If you’re unsure of how to obtain GMP certification in South Africa, you don’t need to worry since Factocert,reputed GMP Consultant, will assist you with all of the necessary steps. Factocert makes GMP registration in South Africa inexpensive and practical. Factocert is easily accessible by visiting www.Factocert.com, where you can chat with an expert, or by sending an email to contact@Factocert.com, where one of our experts will get in touch with you as soon as possible to offer the finest solution on the market.
Our services:
ISO 9001 Certification
ISO 9001 is described as That the international standard that defines requirements for a quality management system (QMS). Organizations utilize the standard to show the capability to consistently provide products and services which meet customer and regulatory demands.
ISO 13485 Certification
ISO 13485 is an International Standard, that specifies the Quality management systems which are involved with medical devices.
ISO 14001 Certification
ISO 14001 is An environmental management system (EMS) has been “that a System and database that incorporates procedures and procedures for training of employees, tracking, summarizing, and reporting of all technical environmental performance information to internal and external stakeholders of a company”
ISO 17025 Certification
ISO/IEC 17025 General requirements for your Competence of testing and calibration laboratories are the chief ISO standard utilized by testing and calibration laboratories. In the majority of nations, ISO/IEC 17025 is your standard for which many labs need to maintain certification to be deemed legally capable.
ISO 22000 Certification
ISO 22000 standards are among those voluntary standards that Specifies a necessity of a food safety management system which may be applied to almost virtually any organization in the food chain beginning from farm to fork. ISO 22000 standards offer a set of prerequisites for your food safety management system and it assists the organization to present the highest quality products for their customers and also makes sure there aren’t any harmful hazards throughout the food processing system.
ISO 45001 Certification
Thinking about ISO 45001 certification an occupational health and safety hazards and preventing injuries at Work is among the important variables or challenges That each organization faces. There are many advantages in regards to ISO 45001 standard one of that raising the brand image of this organization is just one of the significant factor since if your organization is becoming certified it means that the organization has employed and practicing the very top prerequisites that have been accepted internationally for worker safety which dictate bring more confidence and reliability for your customers.
ISO 20000-1 Certification
ISO 20000-1 Certification is an international standard that satisfies the needs for information technology service management systems.
ISO 31000 Certification
ISO 31000 certification is a standard intended for Supplying the need for risk management.
ISO 10002 Certification
The International Standards Organization (ISO) have issued many encouraging standards that could be utilized in combination with the ISO 9000 series, ISO 10000 Series specifically ISO 10002 for customer satisfaction works with ISO 9001, and principles that the goals of the standard throughout the surgical and efficient program of a procedure to advance and implement a code of behavior associated with customer satisfaction.
ISO 22301 Certification
ISO 22301 is about Business Continuity management systems –This standard published by International Organization for Standardization that defines requirements to organize, define, implement, operate, monitor, review, maintain, and continuously enhance a registered management system to safeguard against, decrease the odds of occurrence and prepare for, react to, and recover from disruptive events if they arise.
ISO 50001 Certification
ISO 50001 Energy management systems – Requirements with guidance for use, is An international standard created by the International Organization for Standardization (ISO).
The standard aims to assist organizations in always Reduce their energy usage, and therefore their energy expenses and also their greenhouse gas increases.
ISO 29990 Certification
The aim Of ISO 29990 will be to offer a standard model for quality specialist practice and performance, and also a frequent benchmark for educating service providers (LSPs) and their customers in the design, development, and delivery of non-formal education, training, and advancement
CE Mark
CE marking is among those merchandise certifications which suggest The item could be lawfully sold in Western nations. Each member state must take that the CE marked goods without experiencing any additional blessings or testing about the standard requirements which are insured by the directives.
CE marking means that the maker Must confirm that their products satisfy all of the demands of the directives that need to be put on the item.
Halal Certification
Halal is a phrase from The Quran that means”allowed” or “legal”. Therefore, concerning food, Halal is utilized for food and other consumables which are permissible for ingestion and utilized by Muslims, according to Islamic law, the Shariah.
HACCP Certification
HACCP Certification Is an endorsement system that admits a food business has grown recorded and implemented systems and processes according to HACCP. HACCP stands for Hazard Analysis Critical Control stage and is an internationally proven instrument that will help identify and control food safety hazards that might happen inside the food business.
GMP Certification
Good Manufacturing Practices (GMP) will be the practices needed to conform to the principles advocated by agencies that control the consent and licensing of their manufacture and purchase of food and drinks, cosmetics and pharmaceutical products, nutritional supplements, and medical devices.
GLP Certification
From the experimental (non-clinical) Research stadium, good laboratory practice or GLP has been also a quality system of management controls for analysis laboratories and organizations to guarantee the uniformity, reliability, consistency, reproducibility, quality, and ethics of goods in development for animal or human health (such as pharmaceuticals) through non-clinical safety evaluations; by physicochemical properties via acute to chronic toxicity evaluations.
SA 8000
SA 8000 will be An auditable certification standard that motivates organizations to grow, preserve, and employ socially appropriate practices at work.
VAPT Certification
Vulnerability Assessment and Penetration Testing (VAPT) Describes a wide selection of security testing services made to recognize and help manage cybersecurity exposures.
CMMI Certification
Capability Maturity Model Integration (CMMI) is a process-level advancement training and Evaluation program. Administered from the CMMI Institute, a subsidiary of ISACA, it had been designed In Carnegie Mellon University (CMU).
Our Services
Our Clients










































GMP audit services in South Africa are essential because if you’re facing the GMP audit in South Africa you have to be very careful about every parameter in your organization but when you join hands with Factocert. We as an GMP Consultancy Service provider in South Africa, are tagged up with so many different GMP certification bodies in South Africa.
Benefits of GMP Certification in South Africa
Marketing becomes very easy when you have certification tagged on to your name and which will help you to get into a global market and be a global player.
You will have the edge over your list of GMP companies in South Africa.
Employee satisfaction rates increases which are directly proportional to your employee retention and by retaining your critical employees you will have higher stability as a company
A government will recognize you for having such GMP certification in South Africa
You will be automatically qualifying for any tenders because most of the companies require you to certify for GMP certification in South Africa for participating in tender
Customer satisfaction rates will go high, and you will not have to face any more consequences from your customers or your vendors
Are you looking for
GMP Certification Consultants in South Africa
What else are you waiting for the only step you have to take care of is getting in touch with us, and we would take the best care, and in no time you would be certified for the relevant GMP certification in South Africa you prefer. Most thing companies worry about is GMP service cost in South Africa but let us just tell you that GMP cost in South Africa is not what you should be thinking of because when this certification can give a boost to your organization’s process.
We will make sure that the cost of GMP certification in South Africa is as minimal as possible. To get you GMP Certification Services in South Africa than we assure you 100% guarantee results and we ensure that you will definitely be certified because have 100% success rates till date in getting our customers certified. So get in touch with us as early as possible and get your GMP certification in South Africa at the earliest.
Mail us at contact@factocert.com for quick assistance.




















