GMP Certification In Saudi Arabia
GMP Certification In Saudi Arabia
GMP Certification in Saudi Arabia, Factocert is the GMP Consultants located in Saudi Arabia for providing the GMP Certification in Saudi Arabia, Riyadh, Dammam, Jeddah, Medina, Al Khobar, Mecca, Jazan, Tabuk, Jubail, Buraidah, and many other cities in Saudi, offering implementation of training as well as documentation gaps analyses, registration audit, and templates at a reasonable cost to all businesses to be certified under the Good Manufacturing Practice management system in Saudi Arabia.
What are the steps to get GMP Certification in Saudi Arabia?
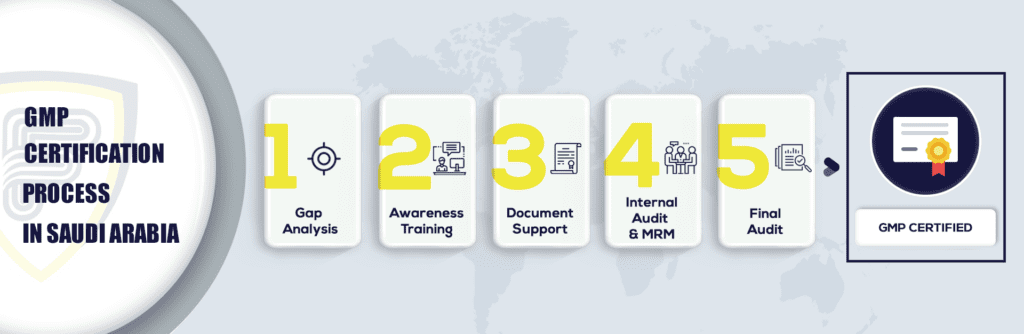

GMP certification in Saudi Arabia is a process-related certification that identifies the need for the best manufacturing methods. This standard applies to every manufacturing sector, which is not a universal standard. The standard is essential to measure your standard against International best practices. Kingdom of Saudi Arabia- KSA the advantages of the standards are the greatest to aid all manufacturing industries, reduce the cost of waste management, customer handling retention of employees, and profit margins.
GMP accreditation services within Jeddah have helped many manufacturing industries improve their image in domestic and international markets. It also allows companies to take advantage of the possibility of expanding the business into international markets, leading to higher profits. GMP registry services in Riyadh have a higher demand due to the benefits of increasing profit margin. It isn’t a requirement but the best practices used by successful businesses around the globe.
GMP Registration in Dammam can help you participate in government tenders, resulting in greater business opportunities within the nation. It will help you boost your confidence levels to compete with your rivals. In this way, defects can be detected in the initial stages of manufacturing, which can save money and expense that would otherwise be lost if they were discovered later on. Using a third party to provide GMP auditing services at Al Khobar is highly recommended, which will help you find more inconsistencies within the system. It is advised to follow the guidelines before applying for certification. Many organizations want to buy the certification without implementing the process, which can have the highest number of negative effects on the company and cause brand damage.
GMP in KSA is The right person to guide you on what is required by the standard. You will also receive all the necessary documentation to meet the requirement for certification. As a company owner, everyone around is focused on running your business and not completing the documentation required for the standard. GMP consultants in Riyadh will provide aid to help you meet the specifications of the standard in order and assist you in getting accreditation. GMP certification in Jubail will allow you to reap the most benefit only if you’re extremely cautious when dealing with suppliers. GMP certification in Saudi Arabia is among the most effective practices being used within the manufacturing and the food, pharmaceutical, and food industries that improve the efficiency of processes within the manufacturing company.
As per GMP accreditation in Jeddah, This is among the most prestigious standards, only known for hygiene and quality. It results in an unintentionally hazardous process that produces products that are without defects and safe for the consumer to utilize the product. GMP certifications in Riyadh assure that the manufacturing facility or process is completely monitored to ensure that the final product will function as designed. It is among the best practices in the industry that is a compliance certificate. Without it, there is no accredited body to provide the accreditation. As a prerequisite to complying with other international standards, including food safety management systems, GMP Certification in Saudi Arabia has become a prerequisite standard for the food industry. It is among the standards being demanded by governments across every country to be able to contracts that the government sector receives.
GMP certification for Al Khobar is a standard created or developed through the collection of all the practices used by all of the successful organizations around the globe. An analysis of the most effective collection practices from the available options is conducted of the most effective out of the best ones. GMP Certification Services in Saudi Arabia These standards have evolved from the quality management system. In line with best practices, some requirements are a quality management system, personal hygiene, factory standards, and contamination control. Under the quality management system, GMP Certification Services in Jeddah lays out the obligatory requirement for an inventory of hazards that must be documented. Hazard inventory is an account or register that records the risks in the organization process. To arrive at an appropriate time to register an incident in the register, you must first go through the hazard analysis, which is the crucial area to concentrate on.
The GMP Certification within Saudi Arabia must undergo a rigorous risk assessment and understand aspects such as critical control areas. In Dammam, GMP Certification requires regular monitoring of the requirements once you know them to prevent contaminated food from exploiting any weaknesses. Corrective actions play a significant part in hazard analysis because they can assist us in understanding what could have been the primary cause of the incident. The best methods for analyzing the root causes would be the fishbone diagram and the five reasons currently being utilized by most organizations around the globe according to the research being conducted and the results presented through GMP Certification Services in Al Khobar. Management responsibilities that are similar to international standards are also important. The standard requires that top management participates in the implementation process.
It ensures that GMP in Riyadh is continually improved. It also offers the option of choosing managerial positions or individuals responsible for implementing the management method and pushing towards achievement. The management must ensure that to attain the GMP certification and registration in Saudi Arabia. Resources play a significant contribution. To ensure that all barriers to implementing these best practices are removed and that an extremely strict and rigorous system has been established for the company’s long-term success, the management should ensure that the essential requirements for qualified and competent resources have been met. GMP Certifications within Saudi Arabia specify the mandatory requirement to determine the skill and competence of the individual or team responsible for the implementation process. The team members must also maintain a matrix that determines their specific skills so they can be instructed accordingly. As an additional document to support this requirement, you must maintain an assessment of the individual’s skills.
As per GMP registration services in Saudi Arabia, training is among the secrets behind the systems that work. Many organizations believe that offering employees training will benefit their personal development. Still, the truth is that training can benefit employers and employees. During the GMP registration process in Riyadh, it is required to document the evidence used for the training provided and a record of the evidence required in the GMP registration process in Jeddah. You can use feedback forms, training registers, and assessments to demonstrate conformity with the standard.GMP registrations in Dammam also mandate evidence of the person who manages the team.
To comply with this requirement, as an organization, you must ensure that the highest management has signed off on the leader’s appointment. The next essential requirement for Saudi Arabia GMP services is to maintain a quality guideline that is the same as the Quality Management System. However, in the latest model of quality management systems. No requirement requires an organization to keep the quality manual. However, the organization implementing this best practice must ensure that the manual is documented and readily available for GMP audits in Saudi Arabia.
You must understand the basic requirements of the manual and then determine the scope of certification, which means determining the boundaries within your organization and the resources or power sources, like technology, location, etc. For a certification audit to be successful, it is important to ensure that information is recorded in the manual and is available as evidence or documentation. GMP certification in Saudi Arabia also demands that the goal be defined according to the best methods. Organizations must make sure that all of the objectives outlined are met.
The price of GMP accreditation for Saudi Arabia will only be determined by a few elements like the extent of certification, the number of person-days required by the Consulting organization or certification body, and accreditation bodies. Based on the industry’s best practices, all individuals’ roles and responsibilities must be identified and documented in the manual. According to the documented procedure, this manual will be an internal document made accessible to the public. It is protected and used only for the organization process.
GMP experts in Saudi Arabia The document must specify the requirement for a standard procedure for documenting the procedures, policies, and other forms. These steps will help the team understand how to draft the document, including its objectives and scope, normative references to terms and definitions, roles and responsibilities, guidelines, procedures, and policies. In Jeddah, GMP is responsible for assisting the organization or team implementing this management system in defining a process that enables the user to know what document is titled or numbering, what version it is in, who is responsible for reviewing and approving the documents, and when it is due for modification are the things that need to be documented in policies. GMP Consultants in Riyadh must ensure that the necessary guidance and education are provided to ensure that the level of competence in the documentation of procedures has been raised.
Dammam GMP must communicate the significance of this standard or procedure, which is nothing more than ensuring that the data is recorded uniformly, following the standard’s requirements. To reduce the time spent on the documentation and implementation of these procedures, GMP experts in Al Khobar should ensure that they have the required templates or a custom template. As part of GMP Certification in Saudi Arabia, the organization must also establish a procedure for handling customer complaints, often neglected by organizations, resulting in brand damage and lost customers. GMP consulting services in Jeddah must emphasize the importance of being accepted as a long-term customer. The purpose of having a procedure for handling customer complaints is to identify the system’s loopholes and take corrective measures to prevent this from occurring again in the future.
GMP in Riyadh It is essential to establish a policy to deal with customer complaints. One of the best ways to do this according to best practices in the industry to satisfy this requirement is to keep a register of complaints. Following the standard procedure, there aren’t any specific requirements regarding managing customer complaints. Organizations worldwide manage customers’ complaints using automated or manual methods. You can pick the method you prefer and feel at ease with you. The GMP Certification for Saudi Arabia has to make sure that there’s an effective process for you to look into complaints being received by your customers. Once you have identified the reason for the issue, you must ensure that the steps taken to correct the issue to ensure that the issue will not happen in the future. Al Khobar GMP Consultant Services ensure that the client is given priority and that customer complaint are resolved to improve and delight the client so that they remain a long-term client.
GMP Consulting Services in Saudi Arabia will also outline the obligatory process to be documented for removing the products in emergencies. It is also referred to as recall in manufacturing industries. GMP Consulting Services in Jeddah must ensure that the recall procedures have been established and documented according to the industry’s best practices. GMP Consulting Services in Riyadh is required to ensure the procedures are documented. Certain elements affect manufacturing or production processes that occur inside the batch number. Every batch number needs to be recorded and tracked. The GMP Certification in Saudi Arabia also has to ensure that each product in a particular batch has a unique number so that the product is recalled in the future when an defective item is manufactured. It is easy for the organization to trace those who have used the service. It will help your company avoid legal problems.
GMP Consulting Services in Al Khobar ensures that your confidence and care for your customers will enhance your company’s image, increasing profits. The GMP Certification consultants Certification within Saudi Arabia specifies the mandatory requirement of having a procedure for tractability. Traceability is among the aspects to be evaluated in importance in most organizations as per the analysis of GMP certification consultants. GMP Certification consultants from Jeddah have put in place a massive system. Still, they could not access the documents and other required evidence. An organization or system with everything in place but cannot show the proof is of no use. As a result, therefore, the consultants of GMP accreditation within Saudi Arabia have to make sure there’s an established procedure and process for tracking the system. According to the GMP Certification within Saudi Arabia, having a master list of records and documents is a best practice in the industry that is suggested for all departments to maintain. It should contain certain areas such as document number, name, approval, review, etc.
GMP Certification for Saudi Arabia has to make sure to cut down on time spent getting the documents back. By including a hyperlink to the document’s reference number within the master list, you will be guided to the relevant procedure or policy with a single click, reducing the time spent locating the document. The GMP Certification requirement in Saudi Arabia also specifies the obligatory requirements for maintaining a process for monitoring your suppliers or vendors. Following industry best practices, it is highly recommended to purchase your vendors.
Among the key factors that directly affect the manufacturing of high-quality products is the quality of raw materials supplied to an organization by GMP consultants in Saudi Arabia. Your vendor plays an important function in manufacturing products that could result in customers’ satisfaction and high-quality products. Thus, you should either order your vendor’s process yourself or hire a company that will assist you in confirming the vendor’s process to guarantee that you receive defective raw materials that will enable you to produce a certain product for customers.
How can I obtain a GMP-Certification within Saudi Arabia?
We are among the most well-known and globally acknowledged consulting firms for international certification. We have experience handling each International Standard in every industry. We are known for our high-quality services to all our customers worldwide. We assure you of a hundred percent customer satisfaction. We believe in assisting our customers gain the maximal benefits from industry best practices and helping them improve continuously. With us, GMP certification within Saudi Arabia is always reasonable for all our customers in all sectors. Our unique implementation methods of international standards have resulted in a clear understanding of the requirement of the bar.
How do I become certified as a GMP expert within Saudi Arabia?
Suppose you’re considering How to obtain GMP approval in Saudi Arabia. In that case, We are always available by writing to us at Contact@factocert.com and supplying us with the details about your business to help us evaluate the need to assist you in obtaining the certification. Our experts can also provide you with the most appropriate solution by answering your questions via our official website at www.Factocert.com. If that doesn’t work, you can contact us at www.Factocert.com with the contact details of your help desk for a phone call with one of our experts. Who needs a GMP Certificate for Saudi Arabia?
GMP certification is required by companies involved in the production of beverages and food, cosmetics, pharmaceutical products, and dietary supplements to comply with regulations and statutes and to gain the trust of customers, consumers, and stakeholders.
How to Obtain GMP Compliance in Saudi Arabia?
Contact Factocert to obtain GMP certification. Share your requirements with Contact@factocert.com to get GMP certification at the best price in the market.
Which is the top GMP consultant within Saudi Arabia?
Factocert provides the top GMP consultation. Please email us at Contact@factocert.com for GMP consultation from our experts at an affordable cost.
Why do businesses need GMP Registration?
GMP registration is essential for businesses to adhere to the required rules and regulations. Many distributors and consumers prefer doing business with registered GMP companies, so the company’s turnover rises.
For More Information Visit: GMP Certification in Saudi Arabia
ISO Certification in Saudi Arabia
Our Clients










































GMP audit services in Saudi Arabia are essential because if you’re facing the GMP audit in Saudi Arabia you have to be very careful about every parameter in your organization but when you join hands with Factocert. We as an GMP Consultancy Service provider in Saudi Arabia, are tagged up with so many different GMP certification bodies in Saudi Arabia.
Benefits of GMP Certification in Saudi Arabia
You will have the edge over your list of GMP certified companies in Saudi Arabia.
Marketing becomes very easy when you have certification tagged on to your name and which will help you to get into a global market and be a global player.
Employee satisfaction rates increases which are directly proportional to your employee retention and by retaining your critical employees you will have higher stability as a company
A government will recognize you for having such GMP certification in Saudi Arabia
You will be automatically qualifying for any tenders because most of the companies require you to certify for GMP certification in Saudi Arabia for participating in tender
Customer satisfaction rates will go high, and you will not have to face any more consequences from your customers or your vendors
Are you looking for
GMP Certification Consultants in Saudi Arabia
What else are you waiting for the only step you have to take care about is getting in touch with us, and we would take the best care, and in no time you would be certified for relevant GMP certification in Saudi Arabia you prefer. Most thing companies worry about is GMP service cost in Saudi Arabia but let us just tell you that GMP certification in Saudi Arabia is not what you should be thinking of because when this certification can give a boost to your organizations process.
We will make sure that the cost of GMP certification in Saudi Arabia is as minimal as possible. To get you GMP Certification Services in Saudi Arabia than we assure you 100% guarantee results and we ensure that you will definitely be certified because have 100% success rates till date in getting our customers certified. So get in touch with us as early as possible and get your GMP certification in Saudi Arabia at the earliest.
Mail us at contact@factocert.com for quick assistance.




















