GLP Certification In Jordan
GLP Certification In Jordan
GLP Certification in Jordan or the Good Laboratory Practice is a top-quality system of administrative controls for the laboratories as well as organizations to make sure the uniformity, reliability, reproducibility, quality, and also the integrity of products in growth for human or animal wellness (consisting of drugs) via non-clinical safety and security tests; from physio-chemical residential properties through acute to chronic toxicity tests.
Factocert provides Good Laboratory Practice (GLP) Certification across Amman, Zarqa, Irbid, Russeifa, and other significant cities in Jordan with implementation, training, auditing, and registration.
GLP manages the organizational process and framework problems of non-clinical health and ecological safety testing of substances and the recording, archiving and coverage of such examinations. It should be used, followed and also confirmed on an ongoing basis.
It is a high-quality system covering the business procedure and problems under which non-clinical research laboratory studies are prepared, accomplished, monitored, documented, reported, and archived. GLP Certification in Jordan ensures the quality and honesty of safety and security examination data submitted to the government for the issuance of research study licenses.
What Are The Steps To Get GLP Certification In Jordan?
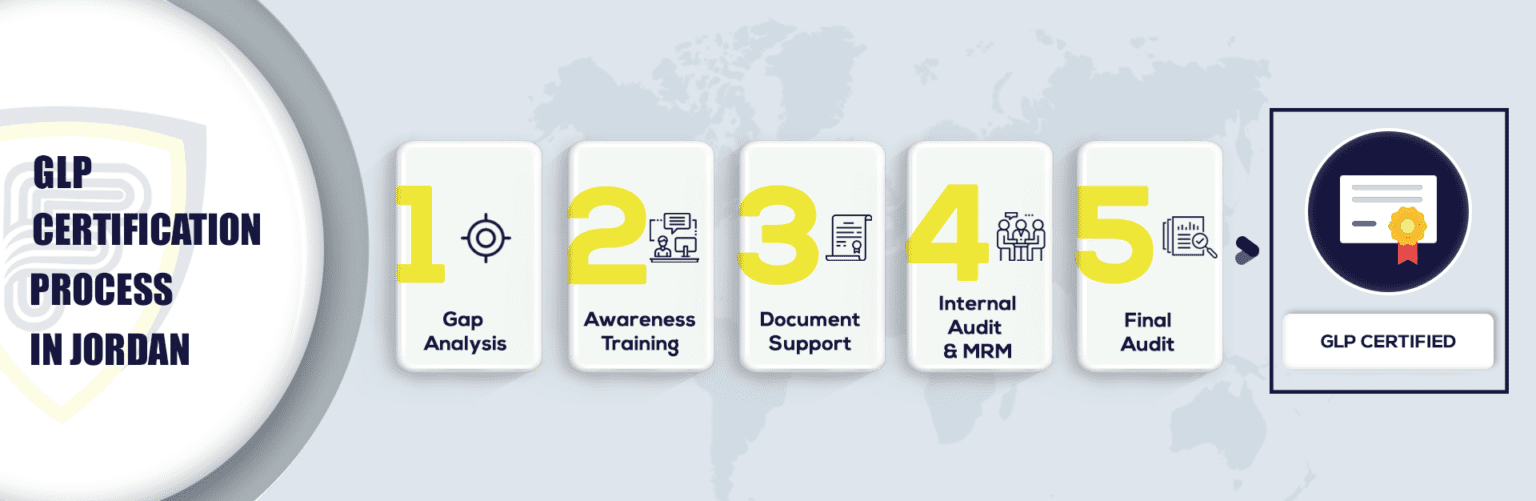

Who Needs to adhere to GLP Certification in Jordan?
- Research studies on animal wellness products: Overdosage researches in the target varieties, animal safety researches in the target varieties, tissue deposit build-up and also depletion researches, and also udder irritability research studies
- When safety studies are done to establish the potential drug-abuse attributes of an examination post are required to be submitted to the FDA as part of an application for a research study or advertising authorization.
- Chemical treatments to characterize the test short article, determine the stability of the examination write-up and its combinations and figure out the homogeneity and focus of test article combinations.
Areas Where GLP Certification Compliance is not required in Jordan:
- Validation tests are conducted to validate the analytical methods used to identify the focus of the examination article in animal cells and medicine dosage types.
- Safety and security research studies on cosmetic products
- Organoleptic analysis of refined foods
- Creating chemical approaches for evaluation or establishing the requirements of an examination post.
- Chemical procedures used to assess samplings (e.g. clinical chemistry).
Interpretation of GLP Terms:
- Testing Facility or Test Facility: The research laboratory performing the non-clinical research.
- Test short article or test Product: The product being examined or tested; the topic of the research study.
- Examination system: these are:
- Any animal, plant, or microorganism to which the test or control write-up is applied.
- Any biological, chemical, or physical system utilized in the study.
- Control Article or Reference Product: An item that is:
- Not the examination article, feed, or water.
- Applied to the examination system.
- Utilized to give a basis for comparison with the test article.
What are the Good Laboratory Practice Guidelines?
Below are Excellent Research laboratory Technique guidelines for the various aspects of a study:
- Employee:
Before the research starts, the testing facility supervisor must assign a study director who will be in charge of the total conduct of the study and its GLP compliance. The testing centre needs to additionally have a Quality Assurance Unit (QAU), which is different from or independent of the screening centre company or administration.
- Center & Tools:
The testing facility needs to supply separation of activities to prevent interference and other disruptions that may compromise the research. There should be separate areas for:
- The receipt, storage space of the test, and control articles.
- The blending of the examination and control posts with a carrier.
- The storage of the test as well as control post mixes.
- The housing of the examination systems.
All tools utilized in the study must be periodically adjusted and also preserved. Records of calibration and upkeep ought to be maintained and provided to operators of tools.
- Report:
Personnel carrying out the research study must recognize the adhering to each examination and control post:
- Identity, pureness, composition, as well as security.
- Date of receipt, expiry date, and storage space guidelines.
- A quantity obtained, as well as the amount utilized.
- Research Study Plan or Procedure:
The study plan or procedure is the master assistance record for the conduct of the research.
It lays out how the study must be done and has the general time routine for the research study and its numerous stages. It additionally includes the method and materials made use of in the research.
Before the study starts, the method must undergo authorization, testimonial, and discussion.
This procedure begins with the research study supervisor preparing the protocol and reviewing its materials with personnel and various other research study personnel. After discussion, the research supervisor must approve the protocol by affixing their dated trademark.
Once the research director has authorized the protocol, it should be reviewed by the QAU, who will undoubtedly assess its conformity with Great Research laboratory Method. The personnel must be advised on their responsibilities in the procedure and get their copies of the protocol.
- Standard Procedure:
Each of the different areas in the testing centre should have Standard Operating Procedures (SOPs), particularly for routine procedures. The testing centre manager must accept SOPs, and the research supervisor must license any deviations from SOPs.
- Final Record:
The last record is ultimately the duty of the research supervisor, who prepares and accepts the record. Critical functions of the final record are:
- A total and accurate account of the conduct of the research.
- Any inconsistency from the desired course of action (such as SOP or procedure).
- Scientific interpretation of outcomes as well as meaningful conversation.
- GLP Compliance Statement by the research director.
- Storage of Records:
Throughout the research program, the study director will certainly be accountable for guaranteeing that all data concerning the research study is recorded and included in safely kept documents. Documents and files, such as the procedure, the last record, and the standard procedure, will certainly be archived at the end of the research.
Only employees authorized by the testing facility manager can access archived records. Additionally, Organizations must log every instance of documents being accessed, eliminated, or returned to the archives. It is also recommended that records in the archives be indexed for arranged access.
- Retention of Records:
The Retention period for archived documents differs depending on national GLP guidelines.
Some of the Good Laboratory Practice Examples:
Below are Primary Good Laboratory Practice examples:
- Put On Personal Protective Equipment (PPE) at any time.
- Communicate with other participants in the study laboratory.
- Join refresher course training and also safety and security workouts.
- Know what you’re doing in any way at times.
- Take notice of unknown scents and substances.
- Use the ideal laboratory equipment for the job or task.
- Consistently clean, adjust, and also keep the equipment.
What are the 10 Principles of Good Laboratory Practice?
The 10 Great Laboratory Practice principles are:
- Examination Facility Organization and Worker.
- Quality Control Program.
- Facilities.
- Apparatus, Product, Reagents.
- Test Equipment.
- Examination and Recommendation Things.
- Standard Operating Procedures.
- Efficiency of the Research study.
- Coverage of Research Study Outcomes.
- Storage and also Retention of Records and Products.
Good Laboratory Practice aims to guarantee the quality and integrity of commercial chemicals and items, which are prerequisites for common acknowledgement of reliable examination information between nations, and to guarantee the reliability and high quality of examination information. Furthermore, human wellness and also security of the environment is an additional goals.
Furthermore, time, expense, and resource savings are aimed at protecting against test repeatings. For more information, you can reach our specialist group from our website:www.factocert.com or get in touch with our team by writing to us at contact@factocert.com and get the answer to all your inquiries.
Why choose Factocert for GLP Certification in Jordan?
Our team of expert GLP Consultants will walk you through the GLP Certification in Jordan Process and make sure that your company’s GLP Certification in Jordan is issued quickly .We will also help you put the latest best practices from the GLP Certification in Jordan guidelines into practice. We will also help you with GLP Consulting and Certification. For more information, visit: GLP Certification in Jordan.
Our Services
Our Clients










































GLP audit services in Jordan are essential because if you’re facing the GLP audit in Jordan you have to be very careful about every parameter in your organization but when you join hands with Factocert. We as a GLP Consultancy Service provider in Jordan, are tagged up with so many different GLP certification bodies in Jordan.
Benefits of GLP Certification in Jordan
You will have the edge over your list of GLP certified companies in Jordan.
Marketing becomes very easy when you have certification tagged on to your name and which will help you to get into a global market and be a global player.
Employee satisfaction rates increases which are directly proportional to your employee retention and by retaining your critical employees you will have higher stability as a company
A government will recognize you for having such GLP Certification in Jordan
You will be automatically qualifying for any tenders because most of the companies require you to certify for GLP Certification in Jordan for participating in tender
Customer satisfaction rates will go high, and you will not have to face any more consequences from your customers or your vendors
Are you looking for
GLP Certification Consultants in Jordan
What else are you waiting for the only step you have to take care about is getting in touch with us, and we would take the best care, and in no time you would be certified for relevant GLP certification in Jordan you prefer. Most thing companies worry about is GLP service cost in Jordan but let us just tell you that GLP cost in Jordan is not what you should be thinking of because when this certification can give a boost to your organizations process. We will make sure that the cost of GLP in Jordan is as minimal as possible.
To get you GLP Certification Services in Jordan than we assure you 100% guarantee results and we ensure that you will definitely be certified because have 100% success rates till date in getting our customers certified. So get in touch with us as early as possible and get your GLP certificate in Jordan at the earliest.
Mail us at contact@factocert.com for quick assistance.
Frequently Asked Questions
What is GLP Certification in Jordan?
The GLP Certification in Jordan stands for International Organization for Standardization. It plays an essential role in maintaining various market sectors’ standards. It starts right from manufacturing an item to providing a product. It is an independent, international organization that develops standards for ensuring the safety, quality, and efficiency of the services and products across Jordan and cities like, Amman, Zarqa, Irbid, Russeifa.
Who Needs GLP Certification in Jordan?
For industries in Jordan, GLP certification might be called for by legislation or contractually. But, even if that’s not the situation, satisfying GLP criteria has many advantages for organizations: Saving money and time by recognizing and resolving persisting issues, Improving system, and process effectiveness.
What are the types of GLP Certifications mandatory in Jordan?
While all the Standards are necessary for different organizations, some of the mandatory Certification Standards in Jordan are:
- GDP Certification: Good Distribution Practices
- GLP Certification: Good laboratory practice
- GMP Certification: Good Manufacturing Practices
- GDPR Certification: General Data Protection Regulation
- SOC 1 Certification: System and Organization Controls 1
- SOC 2 Certification: System and Organization Controls 2
- SA 8000 Certification: Social Accountability
- RoHS Certification: Restriction of Hazardous Substances
What is the cost of GLP Certification In Jordan?
Although the cost of GLP Certification in Jordan depends on the type of GLP Standards, Factocert provides the best GLP Certification services at the most affordable price across Jordan.




















